NEWSLETTER
For inquiries about our products or price list, please leave your email to us and we will be in touch within 24 hours.
INQUIRY NOWCONTACT US
 Building 12, No.309, South 2nd Road, Economic Development Zone, Longquanyi District, Chengdu, Sichuan, China.
Building 12, No.309, South 2nd Road, Economic Development Zone, Longquanyi District, Chengdu, Sichuan, China. amy@enlaibio.com / cynthia@enlaibio.com / edison@enlaibio.com / daisy@enlaibio.com
amy@enlaibio.com / cynthia@enlaibio.com / edison@enlaibio.com / daisy@enlaibio.com +86 (028) 84841969
+86 (028) 84841969
+86 (028) 64841719 +86 135 5885 5404
+86 135 5885 5404
+86 173 9018 3901
+86 158 8456 8590
+86 183 1416 3848
products
Application
© Copyright - 2010-2023 : All Rights Reserved.
Hot Products, Sitemap
amino acid derivatives, amino acids, amino acid, pharmaceutical intermediates, amino acid derivative, pharmaceutical intermediate,
Hot Products, Sitemap
amino acid derivatives, amino acids, amino acid, pharmaceutical intermediates, amino acid derivative, pharmaceutical intermediate,













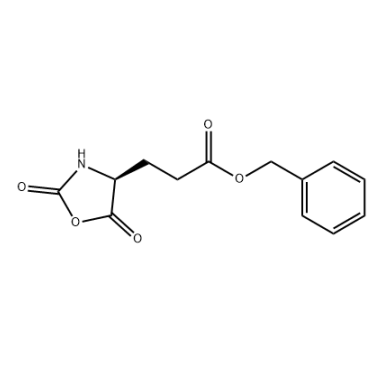
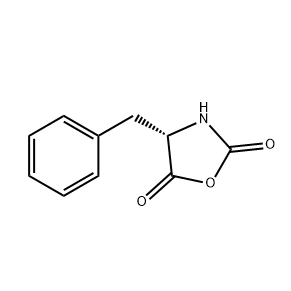
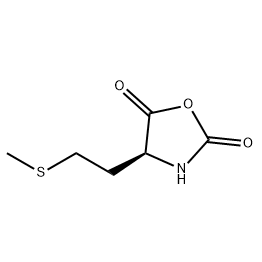
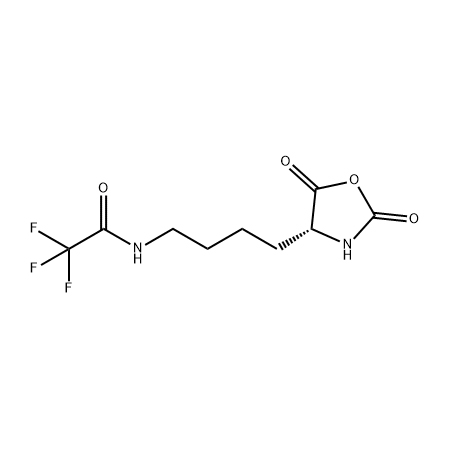





.png)


